Awe-Inspiring Examples Of Tips About How To Find Out Many Valence Electrons An Element Has
![How To Determine The Number Of Valence Electrons In An Element, Ion, Or Molecule [Quick And Easy] - Youtube](https://cdn1.byjus.com/wp-content/uploads/2020/10/Valence-Electrons-2-700x454.png)
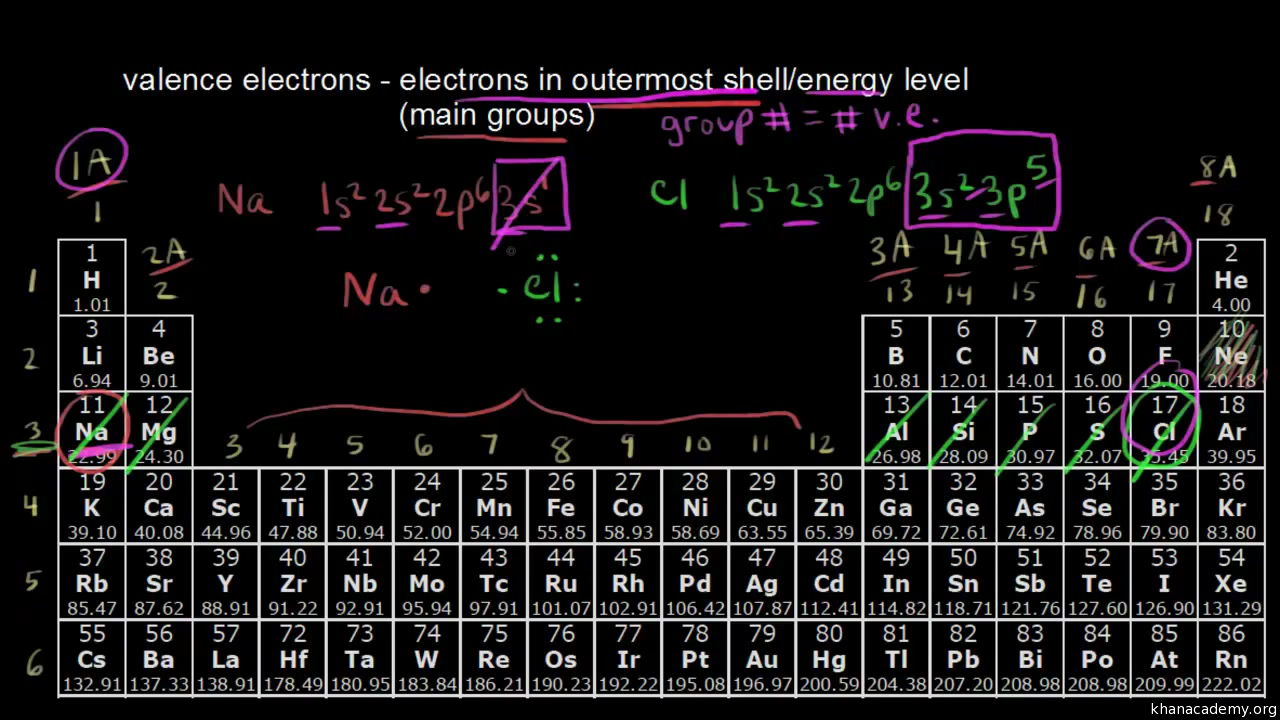
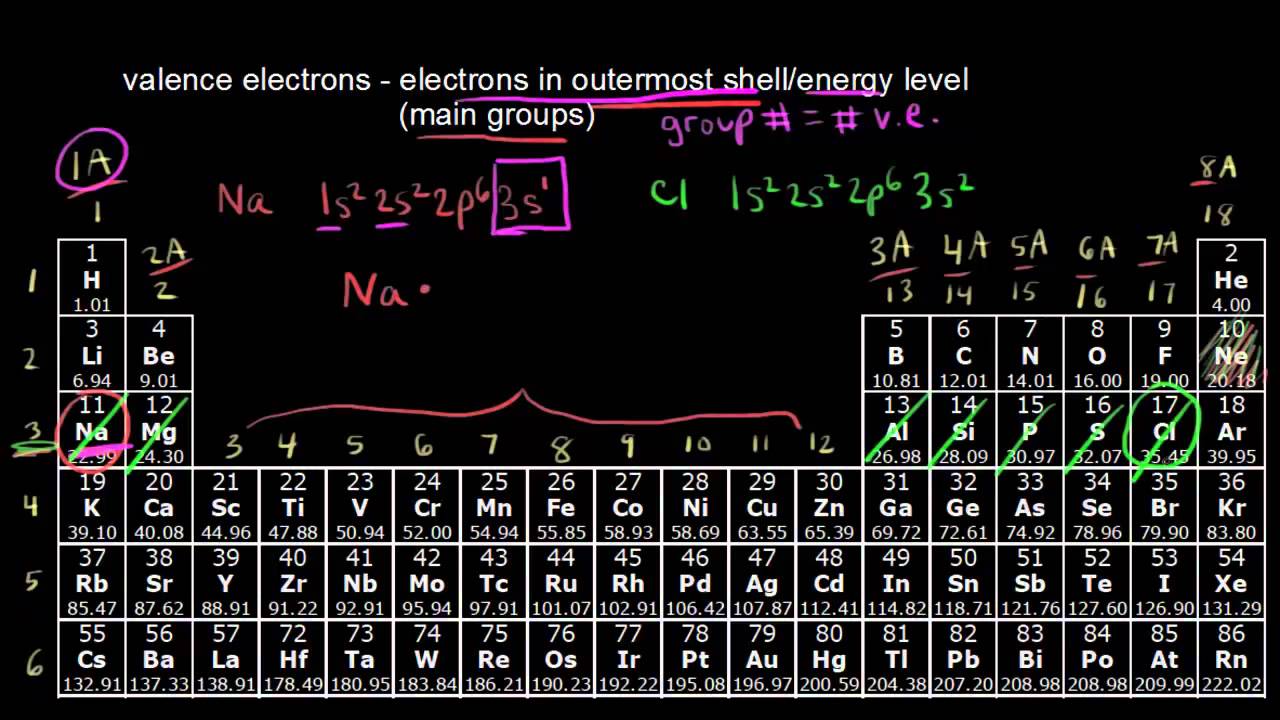
To find how many valence electrons are in an element, simply locate the column number that it is in, and that determines.
How to find out how many valence electrons an element has. Valence shell.for atoms with less than 4. With the help of the periodic table, we can easily see that the atomic. If the charge is positive, there are more protons than electrons.
To find out the atomic number of xenon, we can use the periodic table. The group 1 atoms have 1 valence electron. The easiest way to determine valence electrons is by checking out the element’s place in the periodic table.
For the s and p block elements, the number of valence electrons. And to arrange the electrons, you must know the number of electrons in that. Look at the group that the.
If the charge is negative, electrons are in excess. You can find the number of neutrons if you know the isotope. The periodic table contains rows and columns.
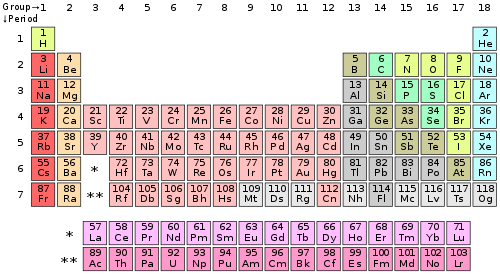
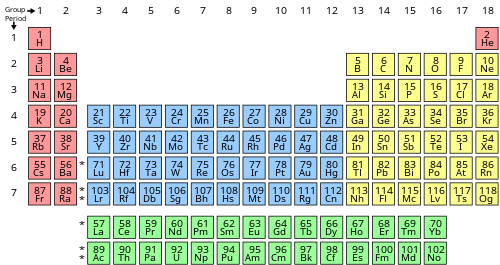
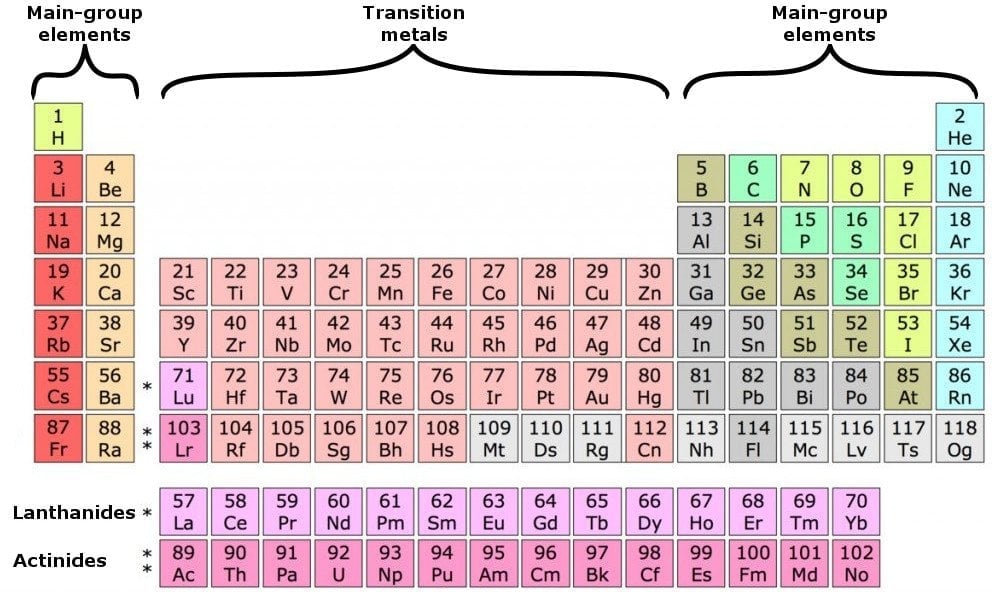
To determine the properties of an element, it is necessary to arrange the electrons of that element. You can get the valence electrons in an atom's electronic arrangement by consulting the periodic table: Looking at the periodic table, atoms have a regularly occurring number of valence electrons based on their group number.
You can use the periodic table to help you determine how many valence electrons an element (specifically, a neutral atom of the element) has. If you look at the periodic table and at the period numbers, that is the number of valence electrons. The position of a chemical element in the periodic table gives a major hint in determining that element’s valence electrons.


![How To Determine The Number Of Valence Electrons In An Element, Ion, Or Molecule [Quick And Easy] - Youtube](https://i.ytimg.com/vi/GEnqFx8MQ5w/maxresdefault.jpg)